Haematopoiesis: the prototypical stem cell system to study proteostasis.
Haematopoiesis is a crucial example of a stem cell hierarchy and represents the ideal system to investigate tissue homeostasis, age-related systemic failure and malignant transformation to cancer. Extensive studies have provided insights into the role of genetics, epigenetics, and the niche in both normal and malignant haematopoietic development. However, our understanding of protein translation, folding, modification and degradation – broadly defined as “proteostasis” – is in its infancy. Considering the dynamic nature of the proteome, both temporally and spatially, the regulation of healthy and malignant stem cells at the protein level remains an exciting novel area of research.
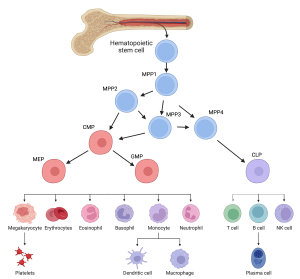
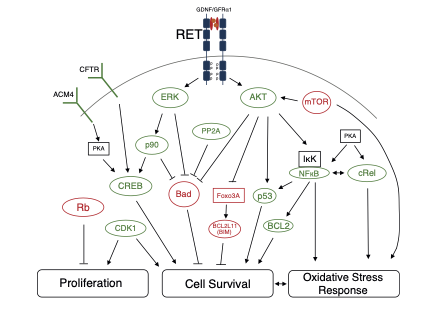
On the left is an example of the haematopoietic hierarchy in mouse bone marrow. All of the blood cells in the body originate from haematopoietic stem cells (HSCs), which themselves produce progeny that slowly commits to producing certain types of blood cells. On the right is an example of a protein signalling map from Grey et al. (2020). Here we demonstrate the complex protein interaction networks that govern expansion of HSCs. When the cell surface receptor RET is activated, a web of interactions downstream changes the functional state of the proteome in HSCs leading to improved proliferation, increased cell survival and activation of the oxidative stress response.
Key Research Priorities
We study the proteome in HSCs, with a particular focus on expansion of HSCs for transplantation. We have demonstrated that loss of the proteostasic regulators CKS1 and CKS2 blocks the ability of HSCs to reform the haematopoietic system upon transplantation (Hemasphere 2023), and by carrying out paired proteomics and transcriptomics demonstrated that there is a disconnect between the proteome and transcriptome in HSCs. An axis we are continuing to study across haematopoiesis and stress responses.
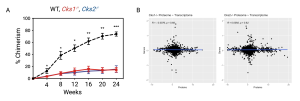
Figure. A. Peripheral blood chimerism of WT recipient mice transplanted with WT (black), Cks1-/- (red) and Cks2-/- (blue) long–term HSCs. B. Correlation between differentially expressed proteins (x-axis) and genes (y-axis) in Cks1-/- and Cks2-/- HSCs.
HSCs require tightly regulated protein kinase signalling cascades, historically most well understood through study of instructive signals (cytokines) from the bone marrow microenvironment. We studied the functional kinome of human HSCs derived from umbilical cord blood to reveal the receptor tyrosine kinase, RET, is a functional target on the cell surface of HSCs which governs a multitude of signalling pathways important for stem cell maintenance and expansion (Blood 2020).
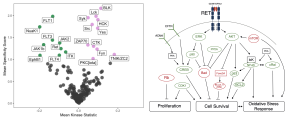
Figure. Left. Differentially active kinases in CD34+CD38- (Green) versus CD34+CD38+ (Violet) umbilical cord blood derived haematopoietic stem and progenitor cells. Right. Key signalling pathways downstream of GDNF/GFRa1-dependent activation of RET on HSCs. Green proteins are hyperactive, red proteins are hypoactive post-GDNF/GFRa1 treatment.
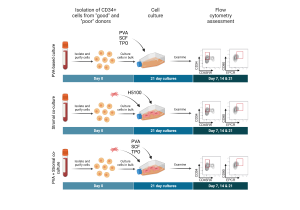
We study HSC expansion on different substrates and media backbones. By combining novel chemically-defined systems (Sakurai et al. 2023) with novel cytokine supplementation (Grey et al. 2020) and classical co-culture systems (Greissinger et al. 2014) we build novel systems to better grow and study human HSCs.